Preconception examination
CarrierTest
Examination of hereditary dispositions that may affect parental fertility and health of the offspring.
One out of five healthy persons on average is a concealed carrier of a mutation of at least one of their genes. When both healthy partners are mutation carriers of the same gene, they can simultaneously transmit the mutation to their offspring. The mutation that is received from both parents is demonstrated by a severe autosomal recessive (AR) disease. Part of the mutations with this type of inheritance is transmitted by women only (genes are located on the X chromosome) and the disease is fully manifested only in their sons, provided that they pass the mutation to them.
AR heredity
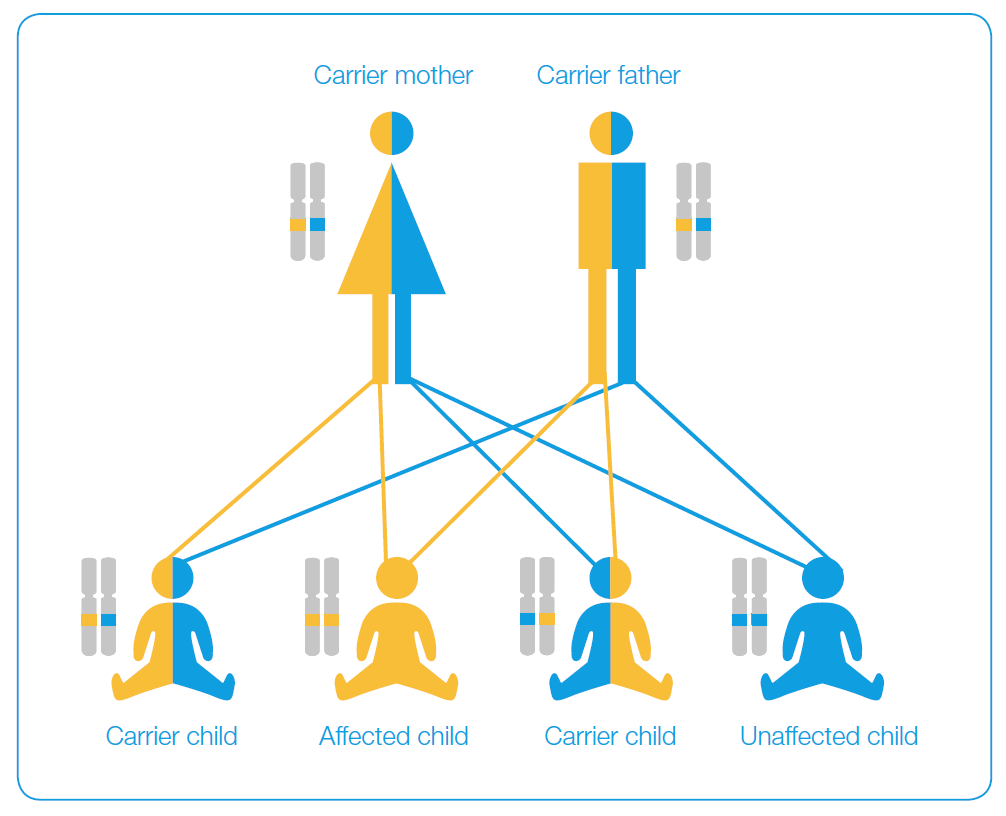
What genes and mutations are investigated with the CarrierTest?
CarrierTest examines hidden transmission of more than 2000 frequent -„key“- mutations of 80 recessive genes that cause more than 75 genetic diseases and conditions which may affect the offspring of healthy carriers. They include cystic fibrosis, spinal muscular atrophy, congenital defects in metabolism (such as phenylketonuria), visual and hearing disorders, musculoskeletal disorders and skin diseases.
Mutations of another group of genes may be of importance for the treatment of fertility disorders (thrombophilia profile, congenital response to hormone treatment of infertility) or they can manifest themselves as a result of lifestyle (hemochromatosis, alpha-1 antitrypsin deficiency).

What are the results of the CarrierTest?
CarrierTest entirely excludes or confirms the presence of a key mutation. However, it does not exclude the transmission of all mutations of investigated genes and the outcome is therefore “residual risk of transmission”. The risk of the offspring being affected is deduced from this risk (degree of genetic compatibility of the couple).
CarrierTest only targets specific mutations in certain genes (for example, when testing thrombophilia profile). The result is in such cases categorical (mutation proven / not proven).
Report on the outcome of CarrierTest is divided into four categories:
- Transmission of gene mutations related to serious diseases in the offspring.
- Mutation of a gene panel which increases the predisposition to blood clotting (thrombophilia profile).
- Response to hormone treatment of infertility (polymorphism of the FSH receptor gene).
The results of testing for recessive mutations and risk for the offspring can be divided into three sections:
-
No mutation was proven in either partner: the risk of occurrence of the tested diseases in the offspring (residual risk) is very significantly reduced.
Mutation carrier of a specific gene was proven only in one partner: residual risk of occurrence of the examined diseases in the offspring is low.
Both partners are carriers of the mutation of the same gene: the risk of occurrence of this disease in the offspring is significantly higher (25% = 1/4).
Measures for mutation carriers of the same gene in both partners with a high risk of occurrence in the offspring:
- Preimplantation diagnosis: embryos resulting from eggs and sperm of the partners from assisted reproductive technology (IVF) are examined and only embryos without adverse combination of mutations are inserted into the uterus of the mother.
- Use of compatible sperm or eggs from donors.
- Prenatal examination of fetal cells following natural conception from chorionic villus sampling (CVS) or amniocentesis (AMC).
- Targeted evidence of hereditary disease and accessible treatment as soon as possible after the birth.
Detection of genetic mutations in the thrombophilia profile, mutations associated with therapeutic response and environmentally-related diseases can be useful for targeted treatment of fertility disorders and adaptation of the lifestyle of future parents.
Limitations, Methodology and List of genes of the CarrierTest (from Dec 2025) can be found here.




